Bathochromic shift (from Greek βαθύς bathys, 'deep'; and χρῶμα chrōma, 'color'; hence less common alternate spelling 'bathychromic') is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency).[1] Because the red color in the visible spectrum has a longer wavelength than most other colors, the effect is also commonly called a red shift.
Bathochromic shift: In spectroscopy, the position shift of a peak or signal to longer wavelength (lower energy). Also called a red shift. A hypsochromic shift is the shift of a peak or signal to shorter wavelength (higher energy). Also called a blue shift.

Hypsochromic shift is a change to shorter wavelength (higher frequency).
Conditions[edit]
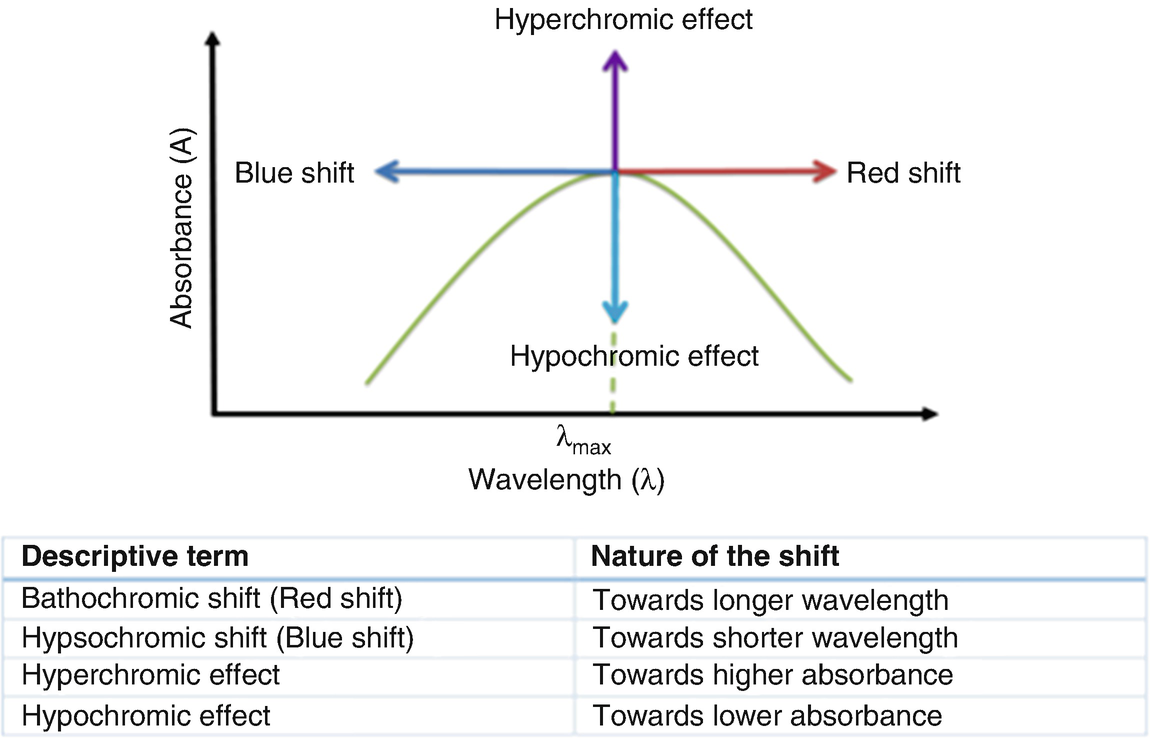
- Dec 29, 2009 Bathochromic, Hypsochromic, Hyperchromic, Hypochromic shifts summarized Changes in chemical structure or the environment lead to changes in the absorption spectrum of molecules and materials. There are several terms that are commonly used to describe these shifts, that you will see in the literature, and with which you should be familiar.
- To understand why conjugation should cause bathochromic shifts in the absorption maxima of chromophores, we need to look at the relative energy levels of the pi-orbitals. When two double bonds are conjugated, the four p-atomic orbitals combine to generate four pi-molecular orbitals (two are bonding and two are antibonding).

Hypsochromic shift is a change to shorter wavelength (higher frequency).
Conditions[edit]
- Dec 29, 2009 Bathochromic, Hypsochromic, Hyperchromic, Hypochromic shifts summarized Changes in chemical structure or the environment lead to changes in the absorption spectrum of molecules and materials. There are several terms that are commonly used to describe these shifts, that you will see in the literature, and with which you should be familiar.
- To understand why conjugation should cause bathochromic shifts in the absorption maxima of chromophores, we need to look at the relative energy levels of the pi-orbitals. When two double bonds are conjugated, the four p-atomic orbitals combine to generate four pi-molecular orbitals (two are bonding and two are antibonding).
It can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism.[2]
A series of structurally-related molecules in a substitution series can also show a bathochromic shift. Bathochromic shift is a phenomenon seen in molecular spectra, not atomic spectra; it is thus more common to speak of the movement of the peaks in the spectrum rather than lines.
- Δλ=λobservedstate2−λobservedstate1{displaystyle Delta lambda =lambda _{mathrm {observed} }^{mathrm {state2} }-lambda _{mathrm {observed} }^{mathrm {state1} }} where λ{displaystyle lambda } is the wavelength of the spectral peak of interest and λobservedstate2>λobservedstate1{displaystyle lambda _{mathrm {observed} }^{mathrm {state2} }>lambda _{mathrm {observed} }^{mathrm {state1} }}
Detection[edit]
Bathochromic shift is typically demonstrated using a spectrophotometer, colorimeter, or spectroradiometer.
See also[edit]
References[edit]
- ^Kamlet, Mortimer J.; Taft, R. W. (1976). 'The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities'. Journal of the American Chemical Society. 98 (2): 377–383. doi:10.1021/ja00418a009. ISSN0002-7863.
- ^Buncel, Erwin; Rajagopal, Srinivasan (1990). 'Solvatochromism and solvent polarity scales'. Accounts of Chemical Research. 23 (7): 226–231. doi:10.1021/ar00175a004. ISSN0001-4842.
Also found in: Medical.
bathochromic
(ˌbæθəˈkrəʊmɪk) adjHypsochromic Vs Bathochromic
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.Link to this page:

